Our Domain of Pharmaceutical Drug Registrations is responsible for preparation, compilation, submission, screening, follow-up & registration of pharmaceutical drugs for human and veterinary use.
Our Domain of Pharmaceutical Drug Registrations handles the following regulatory functions for locally manufactured, Exported and imported drug products: –
- Registration of Pharmaceutical drugs
- Renewal of registered pharmaceutical drugs
- Pre-registration variation of pharmaceutical drugs (Change of brand name, Change/standardization of formulation etc.).
- Post-registration variation of pharmaceutical drugs (Change of Specifications, Change of Source of API/Pellets etc.).
What is CTD Dossier (Form 5) & Its Modules
Pharmaceutical drugs (for human use) can be registered by application on form 5 CTD (Common Technical Document) in DRAP through online system.
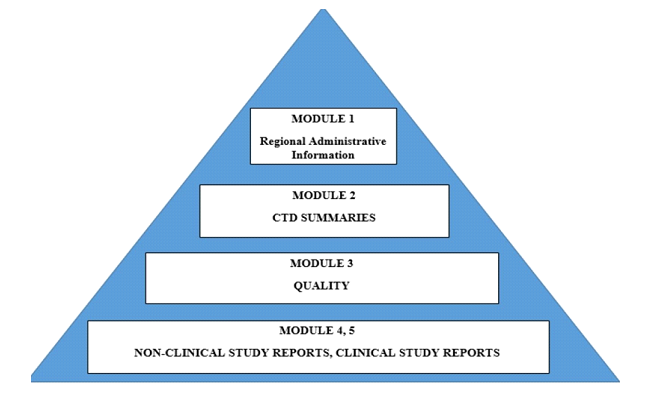
The CTD dossier is internationally agreed format/agreement to assemble all the Quality, Safety and Efficacy information in a common format (called CTD – Common Technical Document). This internationally agreed format used for the preparation of applications regarding new drugs intended to be submitted to regional regulatory authorities. CTD dossier is divided into 5 modules which is mentioned below:
Rules for New Product Registrations in Pharma Sector Related to DRAP
1- Registration Board is the relevant forum for consideration of registration applications.
2- Applicant shall submit drug product registration application form as per Rules 26 of Drugs (Licensing, Registering &Advertising) Rules, 1976 according to product type, as follows: Form 5F–For all types of human drug products* (*Common Technical Document (CTD) (Form 5F)
3- Applicant (manufacturer / importer) shall support drug product registration application with requisite documents and fee.
4- Applicant submits the application to DRAP.
5- These applications are scrutinized and evaluated. After rectification of shortcomings, Registration Board take the final decision. DRAP issues Certificate of Registration of approved drug product to the applicant.
How Fast Regulatory Consultancy Firm expedites product registration processes efficiently:
Expert Guidance Available: Professional expert guidance for CTD Dossier submission
Advisory on pharma regulations: Expert guidance on DRAP Rules & regulations
Customized approach: Customized Services for product registration in Pharma Industry
Improved drug development: Accelerate development, bring drugs to the market.
