Medical devices include any instrument, apparatus, implement, machine, appliance, implant, reagent for in-vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings or animals for one or more of the specific medical purposes of diagnosis, prevention, monitoring, treatment or alleviation of disease.
Medical devices are divided into four classes: A, B, C, and D. It’s a risk-based systems classification. Class A represents the lowest hazard and Class D the highest.
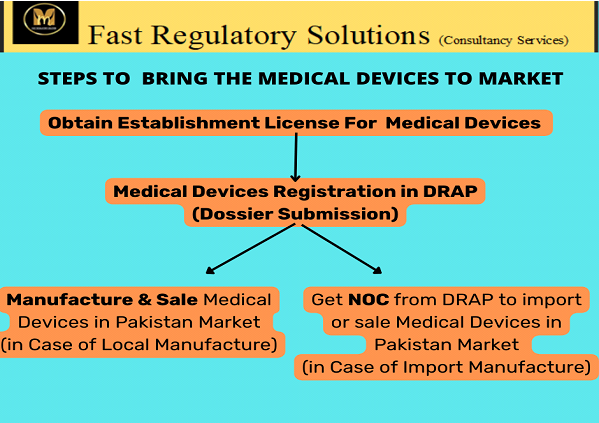
Our Domain of Medical Devices Registrations is responsible for preparation, compilation, submission, screening, follow-up & registration of Medical Devices. Our Domain of Medical Devices Registrations manages the following regulatory functions for locally manufactured, Exported and imported Medical Devices:
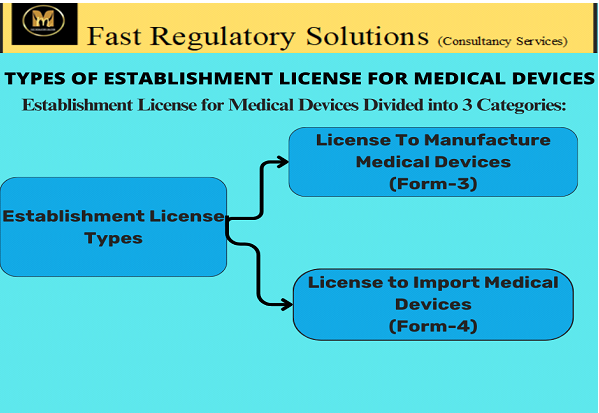
- Application for Grant or Renewal of an Establishment License to Manufacture Medical Devices (Form 1).
- Application for Grant or Renewal of an Establishment License to Import Medical Devices (Form 2).
- Dossier Applications for enlistment or renewal of class-A, B, C, D medical device or accessory or component for local manufacture.
- Dossier Applications for enlistment or renewal of class-A, B, C, D medical device or accessory or component for import.
- Import/Export NOC Applications for custom clearance.
STEPS TO BRING THE MEDICAL DEVICES TO MARKET