Our Domain of Import & Export helps in import/export of therapeutic products of pharmaceutical companies who has valid registration/ marketing authorization of their products with DRAP. These therapeutic products included finished pharmaceutical and biological drug products, active pharmaceutical ingredients (APIs) and drug substances (DS), Medical Devices, and Health & OTC Product (e.g. nutraceuticals, herbals, ayurvedic and homeopathic products, bio-chemic and Chinese products) and their raw materials.
Our Domain of Import & Export manages the following regulatory functions for import/export of therapeutic products of pharmaceutical companies:
- Drug Export License (D.E.L)
- Export NOC of Finished Drugs & Raw Materials
- Drug Import Licence (D.I.L)
- Import Clearance Certificate of Finished Drugs & Raw Materials
Process for NOC:
DRAP has introduced electronic application management system online import and export system (OIES) which enable applicants and regulators to communicate electronically for management of import and export related information and processing of applications related to permission for import and export of therapeutic goods. Process flow Chart for Import/Export NOC is given below:
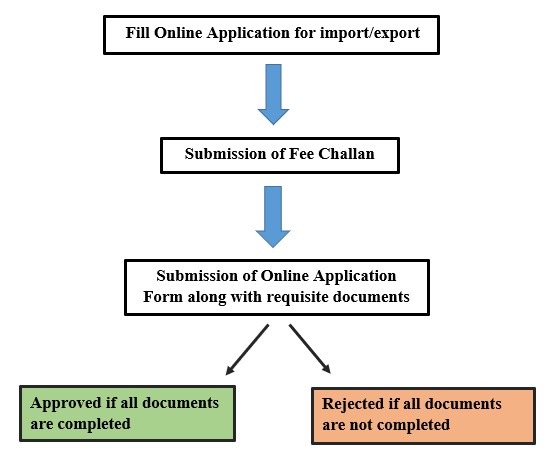
Important Note:
Import and export of any therapeutic goods prohibited under Rule 23 of the Drugs Act 1976 and Schedule II of the DRAP Act 2012 are punishable under Rule 27 of the Drugs Act 1976 and Schedule III of the DRAP Act, 2012. However, unregistered / un-enlisted pharmaceuticals / alternative medicines can be imported by hospitals / institutions under special SRO after obtaining pre-approvals from DRAP. Unregistered / un-enlisted medical devices can also be imported by hospitals / institutions under relevant provisions of Medical Devices Rules, 2017 after obtaining NOC from DRAP. Whereas unregistered / un-enlisted therapeutic goods as donation can be imported under special SRO after obtaining NOC from DRAP.
