Herbal Products are non-prescription products, sometimes known as alternative medicines and health products (Health & HOTC). Vitamins, minerals, herbal products, and homoeopathic preparations are examples of alternative medicine ingredients.
Alternative Medicine means medicinal products which include:
- Indigenous or Unani medicine
- Homeopathic medicines
- Herbal preparation, herbal substance, herbal proprietary medicines, herbal medicinal product.
- Phyto-medicines or any other related products which have been derived from plant, animal or mineral ingredients alone or their combinations but does not contain chemically defined synthetic ingredients.
- Health products means products covered in enlistment rules including food supplements and nutritional products and dietetic foods for infants
Our Domain of Herbal Product Registrations is responsible for preparation, compilation, submission, screening, follow-up & registration of herbal product for human and veterinary use.
Our Domain of Pharmaceutical Drug Registrations handles the following regulatory functions for locally manufactured, Exported and imported Herbal drug products: –
- Application for Enlistment of local manufacturers/ importers (Form-6)
- Application for Post-Enlistment variations of Enlisted local manufacturers/importers.
- Application for Enlistment of products to local manufacturers/importers (Form-7)
- Post-Enlistment variations of Enlisted product of local manufacturers/importers
- Applications for contract manufacturing as local contract manufacturers
- Pre Enlistment variation of products applied for contract manufacturing
- Application for Enlistment of products for contract manufacturing (Form-8)
- Application for FSC (Form-9)
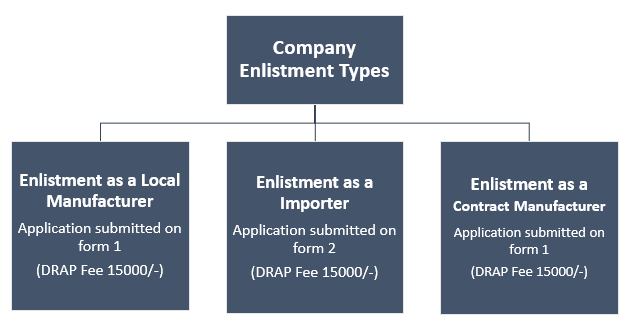
Types of Enlistments Certificates of firm/Company:
Enlistment of company in Nutraceuticals or herbal field Divided into 3 Categories:
How Fast Regulatory Consultancy Firm expedites Herbal product registration processes efficiently:
Expert Guidance Available: Professional expert guidance for Form-3 & Form-4 Dossier submission.
Advisory on pharma regulations: Expert guidance on DRAP Rules & regulations about herbal product enlistment.
Customized approach: Customized Services for product enlistment in Herbal Industry
Improved drug development: Accelerate approval process through Govt. bodies & bring drugs to the market.
